Spirit of pioneering to improve patients' lives
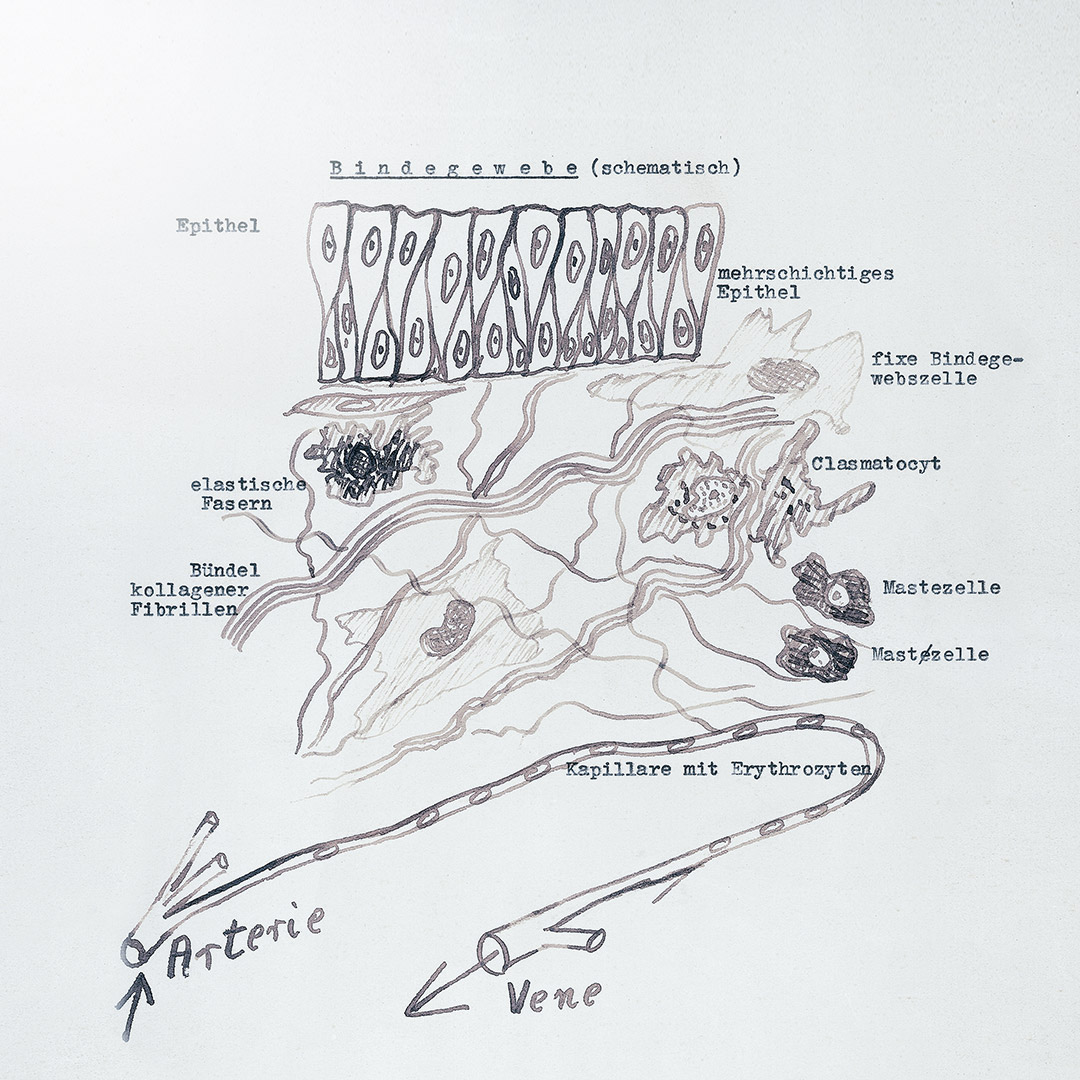
From its early beginnings, Heel has been driven by a spirit of curiosity and pioneering innovation. Established in Berlin in 1936, its founder Dr. Hans-Heinrich Reckeweg was a creative and inquisitive thinker. A physician and homeopath, he not only developed new medicines using purely natural substances. He also pioneered a therapeutic approach based on using those natural medicines to help the body activate its autoregulating mechanisms.
But Reckeweg wasn’t satisfied with just seeing that his medicines and therapies worked – he wanted to know more about them. Scientific research became an integral part of what Heel is about. Supported by our conviction that science-based natural medicine is where the future lies, we want to help spur advances in this field and grow the global knowledge base.
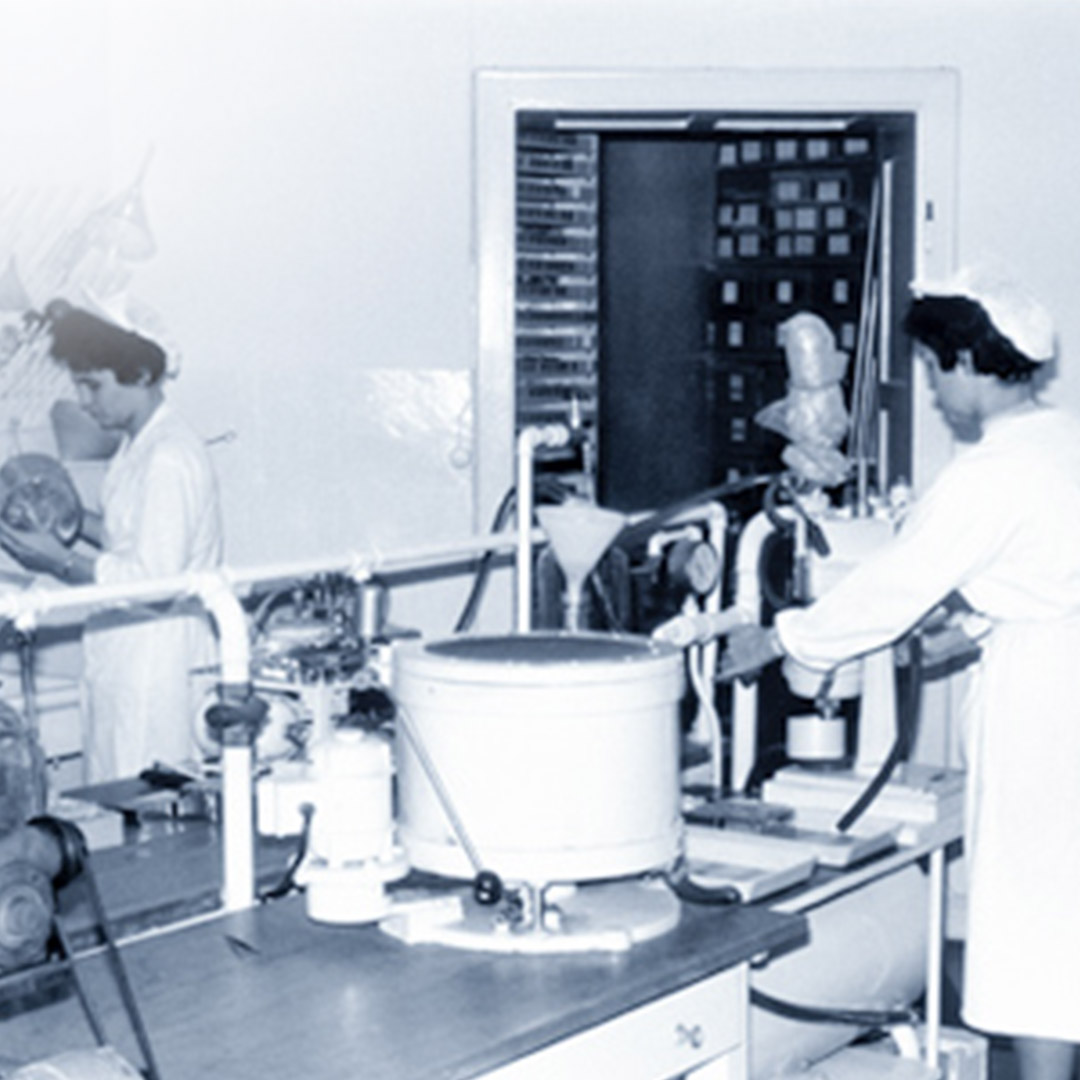
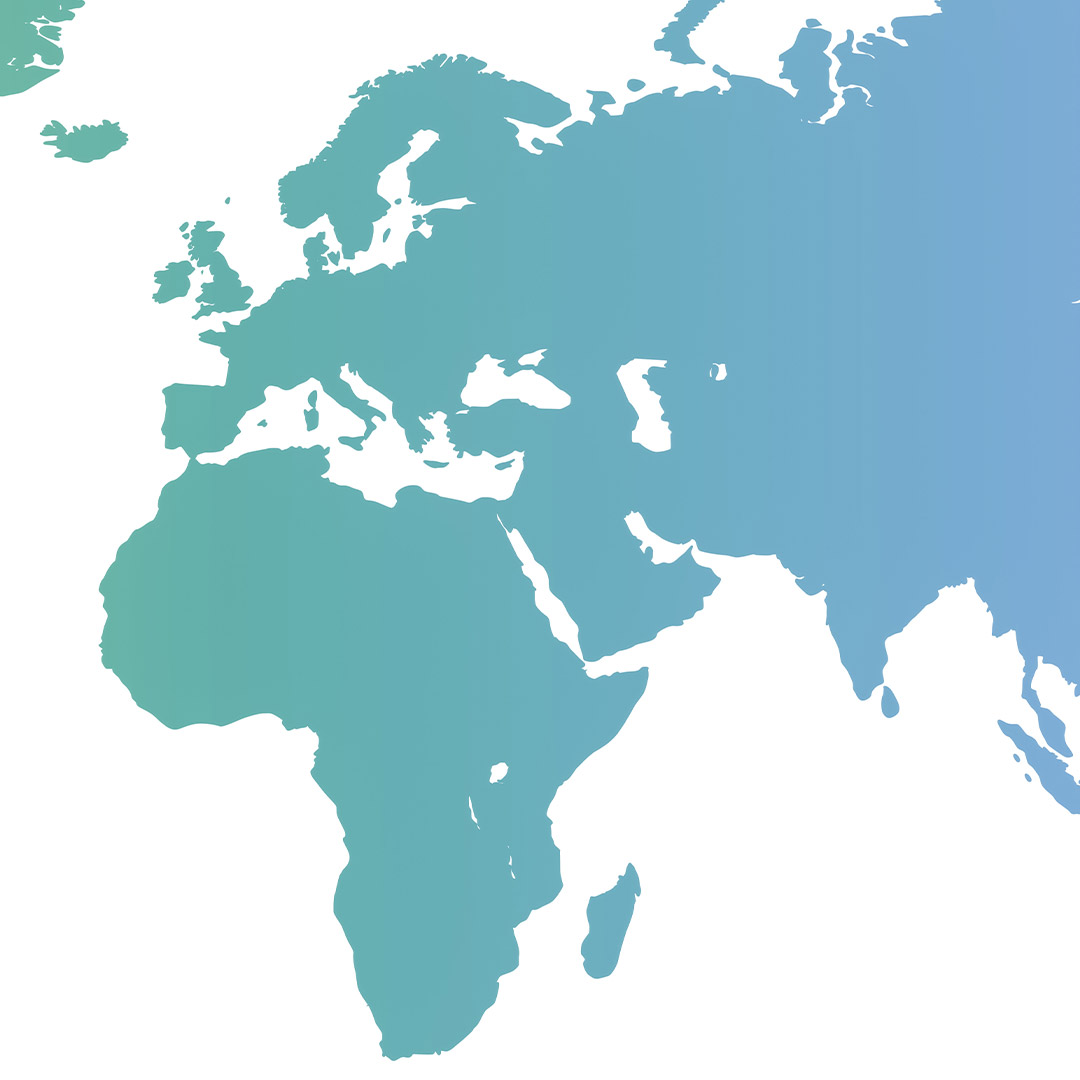
Thanks to this research-driven focus and innovations in the development of products and therapeutic solutions, Heel has continued to grow and thrive. Since then, Heel has been on a steady path of growth and internationalization. From our headquarters in Baden-Baden (Germany), we cooperate with subsidiaries and distribution partners around the world.
Our founder was convinced that natural medicines have a vital role to play in healthcare. His vision paved the way for a modern medicine – an integrative, patient-centered approach to healthcare. Since then, we have been striving for this goal with passion and expertise.